| Lantus | |
|---|---|
| analog by Aventis | |
| long-acting | |
| new molecular entity | |
| U100 | Special-pH 4 |
| Action in dogs: | |
| Line: Aventis | |
| Also known as: Glargine (generic) | |
| Similar to: | |
| Use and Handling: | |
| Shelf Life: 24 months | Type: clear |
| When Opened: 28 days at room temp, up to 6 months when stored in the refrigerator (2C to 8C) | |
| In Pen: 28 days at room temp. | |
| Notes: Protect from light and heat Do Not Freeze, do not refrigerate after opening Do not refrigerate in use cartridges Do not use intravenously [4] Do not use intramuscularly [5] Do not mix with other insulins Do not dilute do not prefill syringe discard if precipitate or cloudiness is seen. | |
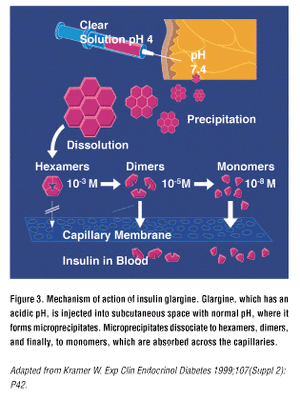
Lantus is the brand name for insulin glargine, an insulin analog made by Aventis [6]. Lantus is a very long-acting insulin (lasting up to 24 hours in humans) that uses pH reactions to form micro-precipitates under the skin, which create a time-release action.
Use in Dogs[]
Dogs normally do not fare well with Lantus. [7][2][8][9] Using it for them can mean using the Lantus as a basal insulin with rapid or fast-acting insulins like Humulin R,Novolin R, Novolog [10] or Humalog as bolus insulin, given at mealtimes. This is how insulin-dependent people use it too. Most dogs receive insulin injections twice a day. Using Lantus would possibly double the number of needed daily injections because of the need for the faster-acting insulin at each meal.
Dr. Rand participated in a study [1] on the effects of Lantus on dogs. The same 9 healthy dogs were tested with Lantus, beef/pork PZI and porcine (pork) lente insulin. It was concluded that Lantus does not lower blood glucose reliably in dogs; there was no consistent peak time or glucose lowering action, and 2 of the 9 failed to have any significant response at all. PZI was shown to significantly lower bg's with longest duration of action (about 19 hours); pork lente insulin began working faster but had less duration (about 10.5 hours).
(A recent study [11] was conducted regarding mixing Lantus and short-acting insulin in the same syringe. The results show that it did not decrease either insulin's effectiveness. There have been more studies like this regarding the combining of Lantus with short-acting insulins. All have been favorable, but the practice has yet to be approved by any regulating body and/or Aventis.)
Compare the study regarding mixing Lantus and short-acting insulin in the same syringe for humans and the information from the Lantus US patient information leaflet on the same procedure in dogs: "When Lantus and regular human insulin were mixed immediately before injection in dogs, a delayed onset of action and time to maximum effect for regular human insulin was observed." [12] The leaflet goes on to say that there was a slight decrease in the bioavailability [13] of both insulins--regular human insulin and Lantus--when both were mixed in the same syringe.
On the basis of the information above, it would appear that Lantus acts differently in dogs than in humans.
Usage and Handling[]
|
Mechanism of Action |
|---|
|
Because of the pH action, Lantus is a bit more sensitive than other insulins -- it needs to have an (acidic) pH of 4 to work, and therefore may not be diluted, mixed with other insulins, or kept overnight in syringes. Lantus and another insulin may be used together in the same patient, but not from the same needle or at the same injection site.
Duration/variability[]
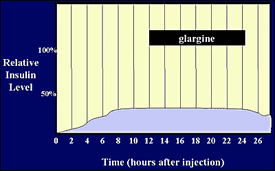
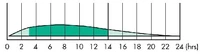
Human time activity profile of insulin glargine (Lantus).
People using Lantus who find that it doesn't last long enough to prevent dawn phenomenon sometimes use a small dose of either Lente or NPH in a separate bedtime injection. This seems to work well for them. [14]
Some people do not have 24 hours duration from Lantus, having what's called an "afternoon phenomenon", where the Lantus taken at dinner the previous night appears to be waning before their next scheduled injection. This occurs even though they are using rapid-acting analog insulins for bolus meal coverage. [15]
Lantus' prescribing information details a study in humans where it was compared to NPH insulin. They found that Lantus has a higher between patient (84%) variability than NPH (78%). [16]
Same-syringe mixing[]
(A recent study [17] was conducted regarding mixing Lantus and short-acting insulin in the same syringe. The results show that it did not decrease either insulin's effectiveness. There have been more studies like this regarding the combining of Lantus with short-acting insulins. All have been favorable, but the practice has yet to be approved by any regulating body and/or Aventis.)
Compare the study regarding mixing Lantus and short-acting insulin in the same syringe for humans and the information from the Lantus US patient information leaflet on the same procedure in dogs: "When Lantus and regular human insulin were mixed immediately before injection in dogs, a delayed onset of action and time to maximum effect for regular human insulin was observed." [12] The leaflet goes on to say that there was a slight decrease in the bioavailability of both insulins--regular human insulin and Lantus--when both were mixed in the same syringe.
Technical Information[]
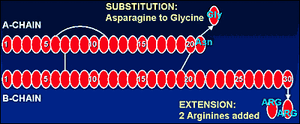
Amino acid structure comparisons of human insulin and insulin glargine (Lantus).
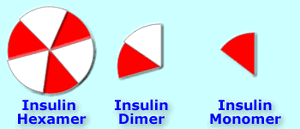
Insulin hexamers must break down into dimers and monomers to be absorbed [18].
The first alteration of human insulin for producing Lantus is found in the A insulin chain at position #21; where normally the amino acid Asparagine is found there, Glycine is substituted. The second modification takes place at the COOH terminus of the B insulin chain, where 2 arginine residues are added. [19]
Addition of the 2 arginines at B-#30 and substituting glycine for the normal asparagine at position #21 on the A insulin chain, keeps the insulin in hexamer form. [20]
Human insulin unaltered has a natural tendency to remain in hexamer form (self-associate); making these changes to the normal human insulin molecule produces an insulin which tends to remain in hexamer form even longer. Inhibiting the breakdown of the insulin hexamers into dimers and monomers means slower onset, peak and longer duration, as absorption takes longer.
|
Long-acting Analogs | |
|---|---|
|
Do not use intravenously. |
|
Lantus insulin Glargine | |
|
Levemir insulin Detemir | |
Analog Insulins amino acid sequences[]
| Amino Acid Sequence of Insulin Preparations [21] | |||||||
|---|---|---|---|---|---|---|---|
| Amino Acid Substitutions | |||||||
|
|
A-Chain Position |
B-Chain Position | |||||
| Source Species |
A-8 | A-10 | A-21 | B-28 | B-29 | B-30 | B-31 B-32 |
| Beef | Ala | Val | Asn | Pro | Lys | Ala | N/A |
| Pork | Thr | Ilc | Asn | Pro | Lys | Ala | N/A |
| Human | Thr | Ilc | Asn | Pro | Lys | Thr | N/A |
| Aspart (Novolog) | Thr | Ilc | Asn | Aspartic Acid | Lys | Thr | N/A |
| Lispro (Humalog) | Thr | Ilc | Asn | Lys | Pro | Thr | N/A |
| Glulisine (Apidra) | Thr | Ilc | Asn | Pro | Glu | Thr | N/A |
| Lantus (glargine) | Thr | Ilc | Gly | Pro | Lys | Thr | Arg |
| Levemir(detemir) | Thr | Ilc | Asn | Pro | Lys | N/A | Myristic Acid |
|
| |||||||
Analog Insulin pharmacokinetics[]
| Pharmacokinetics of Insulin Preparations [21] | |||
|---|---|---|---|
| Insulin Preparations | Onset (hr) | Peak (hr) | Duration (hr) |
| Rapid-Acting | |||
| R/Neutral | 0.5 to 1 | 2.5 to 5 | 8 to 12 |
| Lispro (Humalog) |
0.25 to 0.5 | 0.5 to 1.5 | 2 to 5 |
| Aspart (Novolog) |
0.17 to 0.33 | 1 to 3 | 3 to 5 |
| Glulisine (Apidra) |
0.17 to 0.33 | 1 to 3 | 3 to 5 |
| Intermediate-Acting | |||
| NPH Isophane |
1 to 1.5 | 6 to 14 | 16 to 24 |
| Lente [22] | 1 to 3 | 6 to 14 | 20+ |
| 70/30-30/70 | 0.5 to 1 | 2 to 12 | 24 |
| 50/50 | 0.5 to 1 | 2 to 12 | 24 |
| Novolog 70/30 Mix | 0.25 | 1 to 3 | 24 |
| Humalog 75/25 Mix | 0.25 | 0.5 to 1.5 | 24 |
| Long-Acting | |||
| Ultralente [22] | 6 | 14 to 18 | 18 to 24 |
| PZI [23] | 4 to 6 | 14 to 18 | 24 to 36 |
| Glargine (Lantus) |
1.1 | N/A | 24 |
| Detemir (Levemir) |
0.8 to 2 | N/A | 24 |
These are human activity profiles.
Studies: Lantus in Dogs[]
- Fleeman, Linda, Rand, Jacqueline, 2004 Pharmacodynamic & Pharmacokinetic Comparison of Glargine (Lantus), Protamine Zinc (PZI), Pork Lente Insulin in Dogs University of Queensland
Medical and Vet information about Lantus[]
- Medical Info sheet-Sanofi-Aventis
- Nelson, Richard, 2006 Selecting an Insulin for Treatment of Diabetes Mellitus in Dogs & Cats-page 39 OSU Endocrinology Symposium
- Leeds NHS UK-Insulin Guide
References[]
- ↑ 1.0 1.1 Fleeman, Linda, Rand, Jacqueline (2004). Pharmacodynamic & Pharmacokinetic Comparison of Glargine (Lantus), Protamine Zinc (PZI), Pork Lente Insulin in Dogs. Univeristy of Queensland. Cite error: Invalid
<ref>tag; name "Fleeman" defined multiple times with different content - ↑ 2.0 2.1 Nelson, Richard (2006). Selecting an Insulin for Treatment of Diabetes Mellitus in Dogs & Cats-page 40. OSU Endocrinology Symposium.
- ↑ Abrams-Og, Andrew (2007). Evaluating Insulins for Companion Animals. ACVIM.
- ↑ Mudliar, Sunder, et. al. (2002). Intravenous Glargine (Lantus) & Regular (Neutral) Insulin Have Similar Effects on Endogenous Glucose Output and Peripheral Activation/Deactivation Kinetic Profiles. ADA-Diabetes Care.
- ↑ Karges B, Boehm BO, Karges W. (2005). Early Hypoglycemia After Accidental Intramuscular Injection of Insulin Glargine. Diabetic Medicine (UK).
- ↑ Lantus-Insulin Glargine. Remedyfind.com.
- ↑ Insulins. North American Veterinary Conference (2005).
- ↑ Ruchinsky, Renee, et. al. (2010). Diabetes Management Guidelines for Dogs and Cats-page 7. American Animal Hospital Association.
- ↑ Scott-Moncrieff, Catherine (2009). Canine and Feline Diabetes Mellitus I-page 4. Western Veterinary Conference.
- ↑ Novolog New Drug Application--No Faster Action With Insulin Aspart & Dogs. United States Food and Drug Administration.
- ↑ Chase, Peter. Using the Same Syringe for Lantus and Short-Acting Insulin-pages 3 & 4. Barbara Davis Center for Diabetes.
- ↑ 12.0 12.1 Lantus Patient Information Leaflet-Page 7. Sanofi-Aventis. Cite error: Invalid
<ref>tag; name "Sanofi" defined multiple times with different content - ↑ Bioavailability. Merck Veterinary Manual.
- ↑ Using Lantus & Lente/NPH at Bedtime. Abbott Diabetes Care.
- ↑ Bloomgarden, Zachary (2006). Insulin Treatment and Type 1 Diabetes Topics. ADA-Diabetes Care.
- ↑ Lantus Prescribing Information-US. Sanofi-Aventis.
- ↑ Chase, Peter. Using the Same Syringe for Lantus and Short-Acting Insulin Pages 3 & 4. Barbara Davis Center for Diabetes.
- ↑ Hanas, Ragnar (1999). Insulin Dependent Diabetes-page 5. Children With Diabetes.
- ↑ Alterations to Human Insulin Producing Insulins Glargine, Aspart & Lispro. Clinchem.org.
- ↑ Scientific Discussion-Insulin Glargine-Lantus-Page1. EMEA.
- ↑ 21.0 21.1 Guide to Insulin Preparations. Pharmacy Times.
- ↑ 22.0 22.1 Insulin Pharmacology. Endotext.org. Cite error: Invalid
<ref>tag; name "Endotext" defined multiple times with different content - ↑ Hypurin Protamine Zinc. Net Doctor.co.uk.