The length of time an insulin continues to lower blood glucose.
The four duration categories[1] are:

|
Rapid-acting or Fast-acting insulin begins to work shortly after injection, peaks in about 1 hour, and continues to work for 2 to 4 hours. |
|---|---|

|
Regular or Short-acting insulin reaches the bloodstream 30 minutes to an hour after injection, peaks anywhere from 2 to 3 hours after injection, and is effective for approximately 6-8 hours. |

|
Intermediate-acting insulin generally reaches the bloodstream about 1-2 hours after injection, and is effective for about 8 to 12 hours. |
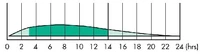
|
Long-acting insulin generally reaches the bloodstream about 2 to 4 hours after injection, peaks 4 to 8 hours later and is effective for about 12 to 18 hours. |
Intro to duration[]
Since activity decays on a half-life curve, there is no actual hard time when duration has ended -- it's a matter of degree. The usual time used for reference is either whatever the manufacturer claims, or the time when blood glucose levels have reached the same point they were at shot time. Any residual effect of the insulin beyond that time is carryover.
Duration varies from species to species -- cats usually experience shorter durations than dogs on most insulins. Duration is also quite individual--can vary from patient to patient and even with the same patient. [2][3] Though it is classed as a long-acting insulin, some humans, like their feline counterparts, do not get this type of duration from r-DNA ultralente insulin. For them, ultralente only has intermediate acting duration. [4]
The insulin time activity profiles [5][6][7] you see are basically averages of blood glucose levels in a test group or groups of patients for a given time period. Some tested patients experienced longer than average duration, while for others it was less than average. Many of the duration test results done by manufacturers of human approved insulins are based on two patient groups--those with diabetes and non-diabetics who agreed to assist in the study. This combined and averaged data is used to generate the time activity profile. It means that there are VERY few with diabetes whose personal insulin activity profile is a perfect replica of those displayed.
It follows then, that onset and peak times found on insulin time activity profiles are just as subject to variability as is duration, since they are all obtained in the manner above.
Duration varies by species and strength, in addition to type. [8] With regard to the non-analog insulins, duration can also be influenced by the size of the insulin dose itself, with larger doses having a stronger effect and a longer duration than smaller ones. [9]
Duration Problems[]
Short Duration
This is a blood glucose graph of a cat who is receiving one shot of Bovine (beef) Lente insulin daily. After the 8 hour point, blood glucose values begin climbing. Interestingly enough, the insulin peak is within acceptable values. This insulin is working for this cat, but he/she is not getting enough duration from only one shot a day. Note: blood glucose values are shown in mmol/l (non-US) measurements.
From what's seen here, it would appear that this is a problem which can be easily solved by increasing from once-daily to twice daily doses of the Bovine (beef) Lente. There appears not to be any Insulin resistance but just a matter of the Intermediate-acting beef Lente not providing adequate control when given only once a day.

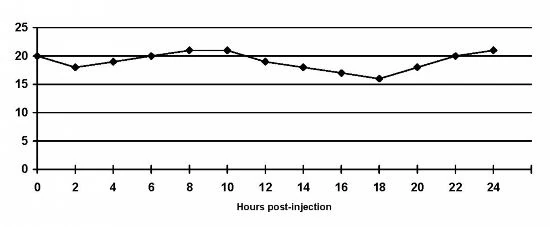
In contrast, let's look at a blood glucose graph of an animal who is being given Lente insulin twice daily, but has resistance to it. (Note: species of animal and insulin not stated. Blood glucose values shown in mmol/l (non-US) measurements.)
You see the cat above with one injection of Bovine (beef) Lente respond to the insulin, but that it simply does not last long enough for him/her. Without knowledge of the species of animal, species of insulin or other information, it is not possible to even speculate as to why the twice daily Lente insulin is not working. There's negligible response to the two Lente insulin shots given in the graph of the animal shown below, and it's provided as an example of resistance/lack of duration only.
Duration By Species[]
With regard to cats and dogs, the closest or perfect amino acid matches to their own native insulin (cats=beef) (dogs=pork) means faster onset, peak and shorter duration, all other things held equal. For both, use of r-DNA/GE/GM insulins would mean a slower onset, peak and a longer duration [10], due to these differences. It's just the reverse for people, [11][12] with the r-DNA/GE/GM insulins acting and peaking faster and having less duration [13] than either beef [12] or pork insulin products. [14][15][16][17]
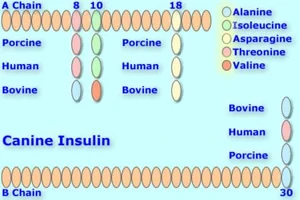
Canine insulin compared with Bovine, Human, & Porcine versions. Up to 2 amino acids differ. Canine and porcine are identical. [18]
Looking at the amino acid matches or differences, we can predict what they mean to duration. Dogs would have the shortest duration from pork insulin, slightly longer from r-DNA/GE/GM human insulin (one amino acid difference at insulin B chain, position #30), [19] but longer from beef, [20][21] with amino acid differences in 2 positions--insulin A chain positions #8 & #10. [22]
In humans, their longest-duration insulin would be beef, [15][23][1] due to the 3 amino acid difference. [24] pork would have slightly more duration for them than r-DNA/GE/GM insulins, because of the 1 amino acid's difference. [25][26][27] One injection of Bovine Ultralente insulin can last up to 40 hours when administered to a human--longer than Lantus; [28] it is also peakless. [29][30][31] When the bovine/porcine Iletin insulins were available, it was possible for some dogs to be controlled on one injection of Iletin I NPH. [32]
Origin, species, or source is very important as it directly affects the absorption, peak and duration of an insulin. [33]
When looking at extending duration in this way, one needs to realize that the amino acid differences produce antibodies; these antibodies are what prevents the insulin from being used so quickly. [19] The possible drawback to extending duration by choosing insulins which are less akin to the native insulin of the diabetic is that the antibodies created by this may cause insulin resistance. [10] Since the antibodies indicate an immune system reaction, all the effects of an overstimulated immune system should be considered. When there is sufficient resistance, duration can decrease instead of increase; at that point, the effectiveness of the insulin is compromised. [21]
Duration By Dose[]
The larger the dose of insulin, the longer it takes to be absorbed [34] and the more duration it will have.[35][36][22]
Duration By Strength[]
Comparing similar insulins with only strength differences means that the U100 strength insulin will have a slower onset, peak and longer duration than an insulin which is U40, U50 or even U80, which is available in some countries, [37] assuming the insulins are otherwise identical. [38][39][22]
An example of comparison for strength would be the two versions of 100% bovine PZI insulin available. Hypurin Bovine Protamine Zinc is a U100 strength insulin; BCP also produces a U100 version of its 100% beef PZI. Both would have the same length of duration.
Switching strengths, however, changes things. Having BCP PZI in either U40 or U50 strength, as opposed to the BCP U100 PZI and Hypurin, means that the U40 and U50 strengths of the PZI BCP makes will have a faster onset, peak and less duration than the two U100 100% beef PZI insulins. The type of insulin is the same-PZI, the species is the same-beef.
The same would be true regarding the insulins of Beta Laboratorios, provided the insulin type and species are the same. Beta's U40 insulins would have a faster onset, peak, and shorter duration than their U80 or U100 insulins, and their U80 strength insulins would have a faster onset, peak and less duration than Betasint insulins in U100 strength.
Less strength=speed[]
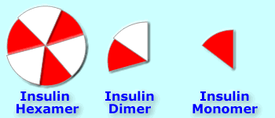
Insulin hexamers (as injected) must break down into dimers and monomers to be absorbed [40].
The hexamers of insulin tend to associate with each other (stay together) when coming in contact with zinc in the bloodstream; [41] they cannot be readily absorbed while they remain this way; this is also how endogenous insulin is stored in the bodies of those who don't have diabetes--in hexamer form. [42]
Diluting insulin into U40 strength forces them into dissociating (staying apart from each other, and becoming dimers and monomers), which means they are absorbed [43][44] better and more rapidly. An easy way to visualize this might be to think of a whole pie, slicing it into 6 pieces, then putting each piece on a separate plate. The pie can't be eaten until it's cut and everyone has a piece on his or her plate.
While the body stores insulin in hexamer form, [42] a normally working pancreas secretes insulin in monomer form, so there's no formation of hexamers by self-association and nothing to break down; the monomer insulin is ready to work .[45]
The less strength an insulin has, the better it is absorbed. [9][44][46] Better or faster absorption means a faster onset and peak but shorter duration. Using a less than U100 strength insulin is on the same principle as rapid-acting analog insulins. The analog insulins have their amino acid sequences altered to produce a faster onset and peak with shorter duration. [47] Diluting insulin from U100 strength does the same thing without altering the molecule. [22]
The fact that U40 insulin has a similar pharmacokinetic profile to rapid-acting analog insulin, has not been lost on the German Institute for Health Care Quality (government department). They proposed a cost-cutting move which would limit prescription coverage of the rapid-acting analogs in Germany. [48]
More strength=duration[]
Humulin R U 500 strength. Note that the vial is 20 ml--twice the size of the standard 10 ml ones and the striping on the carton to alert users to the fact that this insulin is 5 times the strength of standard U 100 insulins [49].
It is also possible to delay the absorption (thus increase the duration) of an insulin by increasing the strength of its concentration. [22] U500 insulin, [50] which is five times more concentrated than U100, [51] has been available through both Lilly and Novo Nordisk (Note: Their similar product is U400 strength insulin [52]) by special order for many years. [53] The insulin's main use is for people with extreme insulin resistance, and is commercially available only in R/Neutral type.
Though it is R/Neutral-type insulin, U400 & U500 insulins [54] have a pharmacokinetic profile more like NPH insulin than U100 R/Neutral. [15][49]
Since there are no additives such as suspensions to alter R/Neutral insulin's action, the strength of the insulin formula hinders its breakdown into dimers and monomers, thus making it much slower-absorbed than U100 and lesser strength insulins. [55][56][57]
In cases of severe insulin resistance, using a much higher concentration of insulin appears to "negate" the effects of immune-related insulin resistance.
The studies at the link below shows that there was no difference regarding antibodies when these patients were transferred from Iletin II NPH at U100 strength to a form of Iletin II R at U500 strength. However, the stronger insulin reduced their insulin needs from 33-75%. [58][59]
Whether the insulin is slowed by the size of its crystals, by its suspension, by the strength of its concentration, or has been designed to release slowly--all of these are different mechanisms with the same goal in mind--to keep the insulin in hexamer form (thus preventing its fast absorption) as long as possible.
If you're having a hard time visualizing the role of strength regarding duration in an insulin, perhaps thinking of the following might help. If you had two stones, one which was pebble sized and the other which was somewhat larger, think about what it would take to break them down into small pieces with a hammer. It would take many more hammer blows to break the larger stone (U100 strength) into small pieces than for the pebble (U40 or U 50 strength); the duration of your work to shatter the larger one would be longer than for the smaller of the two. ![]()
References[]
- ↑ 1.0 1.1 Insulin Therapy. RxEd.org. Cite error: Invalid
<ref>tag; name "Rx" defined multiple times with different content - ↑ Insulin for Cats. BD Diabetes.
- ↑ Insulin Treatment-Individual Factors. Patients Up To Date.
- ↑ Diabetes Forecast-page 2. American Diabetes Association (2006).
- ↑ How Insulin Time Activity Profiles Are Created. Boulder Medical Center.
- ↑ Goeders LA, Esposito LA, Peterson ME. (1987). Absorption Kinetics of Regular (Neutral) & Isophane (NPH) Insulin in the Normal Dog. Domestic Animal Endocrinology.
- ↑ Woodworth JR, Howey DC, Bowsher RR. (1994). Establishment of Time-Action Profiles for Regular (Neutral) NPH (Isophane) Insulin Using Pharmacodynamic Modeling. Diabetes Care.
- ↑ Hirsch, IB, Farkas-Hirsch, R. (1993). Type I Diabetes and Insulin Therapy. Nursing Clinics of North America.
- ↑ 9.0 9.1 Hanas, Ragnar (1999). Insulin-Dependent Diabetes-page 6. Cite error: Invalid
<ref>tag; name "Hanas6" defined multiple times with different content - ↑ 10.0 10.1 Bruyette, David (2001). Diabetes Mellitus: Treatment Options. WSAVA 2001. Cite error: Invalid
<ref>tag; name "Bruyette" defined multiple times with different content - ↑ Duration of GE & Animal Insulins in Humans. Pharmacorama.
- ↑ 12.0 12.1 Marre M, Tabbi-Anneni A, Tabbi-Anneni H, Assan R. (1982). Comparative of NPH (Isophane) Human & Porcine (Pork) Insulin in Diabetic Subjects. Diabetes Care. Cite error: Invalid
<ref>tag; name "Marre" defined multiple times with different content - ↑ Galloway JA, Peck FB Jr, Fineberg SE, Spradlin CT, Marsden JH, Allemenos D, Ingulli-Fattic J. (1982). New Patient & Transfer Studies-GE Duration Shorter Than Animal Insulins in Humans. Diabetes Care.
- ↑ Richard JL, Rodier M, Cavalié G, Mirouze J, Monnier L. (1984). Human & Porcine NPH (Isophane) Insulins Unequally Effective in Diabetic Patients. Acta Diabetologica Latina.
- ↑ 15.0 15.1 15.2 Questions About Insulin. Diabetes-World. Cite error: Invalid
<ref>tag; name "Questions" defined multiple times with different content Cite error: Invalid<ref>tag; name "Questions" defined multiple times with different content - ↑ People Have Faster Onset & Less Duration with GE Insulin than Either Beef or Pork. ChildrenWithDiabetes-Ask the Diabetes Team.
- ↑ GE Insulin of Shorter Duration Than Animal Insulin in Humans. Diabetes Health.
- ↑ Caninsulin-Product Information. Intervet.
- ↑ 19.0 19.1 Brooks, Wendy C.. The Hard to Regulate Diabetic Dog. Veterinary Partner.
- ↑ Horn, B., Mitten, RW. (2000). Evaluation of an Insulin Zinc Suspension for Control of Naturally Occurring Diabetes Mellitus in Dogs-page 3. Australian Veterinary Journal.
- ↑ 21.0 21.1 Nelson, Richard (2006). Selecting an Insulin for Treatment of Diabetes Mellitus in Dogs & Cats-page 40. OSU Endocrinology Symposium.
- ↑ 22.0 22.1 22.2 22.3 22.4 Heinemann, Lutz (January 2008). Variability of Insulin Action:Does It Matter?-page 40 (4 of 9). Insulin Journal.
- ↑ Hildebrandt P. (1991). Subcutaneous Absorption of Insulin in Insulin-dependent Diabetic Patients. Influence of Species, Physico-chemical Properties of Insulin and Physiological Factors. Danish Medical Bulletin.
- ↑ Marre M, Tabbi-Anneni A, Tabbi-Anneni H, Assan R. (1982). Comparative of NPH (Isophane) Human & Porcine (Pork) Insulin in Diabetic Subjects. Diabetes Care.
- ↑ Richard JL, Rodier M, Cavalié G, Mirouze J, Monnier L. (1984). Human & Porcine NPH (Isophane) Insulins Unequally Effective in Diabetic Patients. Acta Diabetologica Latina.
- ↑ Type 1 Diabetes Mellitus & Use of Flexible Insulin Regimens. American Family Physician (1999).
- ↑ Owens DR, Vora JP, Heding LG, Luzio S, Ryder RE, Atiea J, Hayes TM. (1986). Human, Porcine and Bovine Ultralente Insulin: Subcutaneous Administration in Normal Man. Diabetic Medicine.
- ↑ Montgomery, D. A. D.. Modern Insulin and Insulin Therapy-page 2. Royal Victoria Hospital-Belfast.
- ↑ Rizza, RA, O'Brien, PC, Service, FJ (1986). Use of Beef Ultralente for Basal Insulin Delivery. Diabetes Care-American Diabetes Association.
- ↑ Selam JL, Turner D, Woertz L, Eichner HL, Lauritano A, Charles MA. (1989). Comparison of the Safety and Effectiveness of Human and Bovine Long-acting Insulins. Diabetes Research.
- ↑ Background of the Invention. Free Patents Online.
- ↑ Dowling, Patricia M. (September 1995). Insulin therapy for dogs and cats. Canadian Veterinary Journal.
- ↑ Diabetes Forecast-page 3. American Diabetes Association (2006).
- ↑ Bernstein, Richard. The Law of Small Numbers Part 2. Diabetes In Control.
- ↑ Schaer, Michael (2008). Diabetic Phenomena. WSAVA.
- ↑ Fischer U, Freyse EJ, Jutzi E, Besch W, Raschke M, Höfer S, Albrecht G. (1983). Absorption rates of subcutaneously injected insulin in the dog as calculated from the plasma insulin levels. Diabetologia.
- ↑ Hildebrandt P, Sestoft L, Nielsen SL. (1983). The Absorption of Subcutaneously Injected Short-Acting Soluble Insulin: Influence of Injection Technique & Concentration. Diabetes Care.
- ↑ Sindelka G, Heinemann L, Berger M, Frenck W, Chantelau E. (1994). Effect of insulin concentration, subcutaneous fat thickness and skin temperature on subcutaneous insulin absorption in healthy subjects. Diabetologica.
- ↑ Hanas, Ragnar (1999). Insulin-Dependent Diabetes.
- ↑ Hanas, Ragnar (1999). Insulin Dependent Diabetes-page 5. Children With Diabetes.
- ↑ Insulin. Islets of Hope.
- ↑ 42.0 42.1 Insulin. Wikipedia.
- ↑ Sindelka G, Heinemann L, Berger M, Frenck W, Chantelau E. (1994). Effect of insulin concentration, subcutaneous fat thickness and skin temperature on subcutaneous insulin absorption in healthy subjects. Diabetologica.
- ↑ 44.0 44.1 Hirsch (1999). Type 1 Diabetes Mellitus & Use of Flexible Insulin Regimens. American Family Physician. Cite error: Invalid
<ref>tag; name "Family" defined multiple times with different content - ↑ Abrams-Ogg, Anthony (2007). Chemistry and Pharmacology of Therapeutic Insulin Preparations. ACVIM.
- ↑ Hildebrandt P. (1998). Subcutaneous Absorption of Insulin in Insulin-dependent Diabetic Patients. Influence of Species, Physico-chemical Properties of Insulin and Physiological Factors. Danish Medical Bulletin.
- ↑ Polaschegg E. (1998). Effect of physicochemical variables of regular insulin formulations on their absorption from the subcutaneous tissue. Diabetes research and clinical practice.
- ↑ Rapid-acting insulin analogues in diabetes mellitus type 1: Superiority not proven. IQWiG-German Institute for Quality and Efficiency in Health Care (6 June 2007).
- ↑ 49.0 49.1 U-500 Insulin. Diabetes Spectrum (2009). Cite error: Invalid
<ref>tag; name "Spectrum" defined multiple times with different content - ↑ Resource Guide. American Diabetes Association (2005).
- ↑ Humulin R 500. Revolution Health.
- ↑ Cochran, Elaine (2005). Use of U500 Insulin in Patients With Extreme Insulin Resistance. Diabetes Care.
- ↑ Diabetes Forecast-page 4. American Diabetes Association (2006).
- ↑ U400 Insulin. Diabetes Monitor (September 2008).
- ↑ Jørgensen KH, Hansen AK, Buschard K. (2000). Five Fold Increase of Insulin Concentration Delays the Absorption of Human Insulin Injections in Pigs. Diabetes Research & Clinical Practice.
- ↑ Lane WS. (2006). Use of U500 R Insulin by Continuous Insulin Infusion (Insulin Pump) in Patients With Type 2 Diabetes & Severe Insulin Resistance. Endocrine Practice.
- ↑ Humulin R-U500 Patient Leaflet. Eli Lilly.
- ↑ Nathan DM, Axelrod L, Flier JS, Carr DB. (1981). U500 Insulin in the Treatment of Antibody-Mediated Insulin Resistance. Annals of Internal Medicine.
- ↑ Baumann G, Drobny EC. (1984). Enhanced Efficacy of U500 Insulin in the Treatment of Insulin Resistance Caused by Target Tissue Insensitivity. American Journal of Medicine.
See also absorption, peak, onset, carryover and Insulin.
More Information[]
- Variability of Insulin Action-Does it Matter Heinemann, Lutz, 2008, Insulin Journal
- Randomized Controlled Clinical Trial of Glargine Versus Ultralente Insulin in the Treatment of Type 1 Diabetes Diabetes Care-2005