| Iletin I Ultralente | |
|---|---|
| Ultralente by Eli Lilly | |
| long-acting | |
| Bovine 85% Porcine 15% | |
| U100 | Zinc |
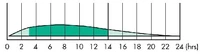
Action in dogs:
| |
| Line: Iletin | |
| Also known as: | |
| Similar to: | |
| Use and Handling: | |
| Shelf Life: 24 months | Type: cloudy |
| When Opened: 28 days room temp. | |
| In Pen: N/A | |
| Notes: Protect from light and heat Do Not Freeze, Re-suspend Do not use if product does not re-suspend Do not use intravenously [1] Do not mix with non Lente-type insulins | |
|
Note that some countries have the brand name in U40 strength. | ||
An ultralente [2] 85% beef, 15% pork insulin formerly made by Eli Lilly.
What Ultralente Is Not[]
No Lente-type insulin regardless of species can contain any NPH/isophane insulin [3] or any R/Neutral insulin. [4][5]
Both are chemically impossible: the phenol preservative present in NPH/isophane alters the action of Lente-type insulins, creating a mixture with an approximate action of R/Neutral. [6][7]
The zinc suspension of Lente-type insulin binds R/Neutral, causing the short-acting insulin to slow, losing its short-acting effect. [8][9]
Before the invention of VetPen, Lente-type insulins could not be dispensed in pen or cartridge form because the glass ball formerly used to mix the insulin in these devices shattered the Lente crystals.[10]
Combining Lente Family Insulins[]
Insulin manufacturers [11] indicate that R/neutral and semilente, Lente, ultralente insulins are able to be combined in the same syringe, but only just before injection. In pre-filled syringes, the zinc suspension of the Lente-type insulins binds the R/neutral, causing it to lose its short-acting effect. Various studies have documented this, and some doctors advise against using R/neutral in the same syringe with the Lente family of insulins. [5][12][13][14]
|
None of the Lente family of insulins (semilente, Lente, Ultralente) can be combined with [15] NPH/isophane insulins. The phenol preservatives present in NPH-type insulins alters the Lente-types to the point where they become a close approximation of R/neutral, with regard to action. [14] Keeping the phenol preservatives in mind, all protamine-suspended insulin mixes would be "off limits" regarding same syringe mixing with any Lente-type insulins. [14] | ||
|
Ultralente Insulins | |
|---|---|
|
Long acting Non-soluble |
| Humulin U, Humulin Zn, Humulina Ultralenta Humuline Ultralong, Umuline Zinc Humulin UL, Huminsulin Ultralong (No longer Produced.) Novolin U, Ultratard Humulin Ultralenta, Humulin Ultralente (No longer produced.) | |
| Iletin Ultralente [16] (No longer produced.) Ultratard [17] (No longer produced.) | |
| Iletin I Ultralente (No longer produced.) | |
References[]
- ↑ Maddison, Jill E.,Page, Stephen W.,Church, David B. (2008). Small Animal Clinical Pharmacology. Saunders Ltd..
- ↑ Dumitriu, Severian (2001). Polymeric Biomaterials, Revised and Expanded 1104. CRC Press.
- ↑ Combining Lente-type Insulins with Phenol-Preserved Insulins. National Federation for the Blind.
- ↑ Lente Zinc Suspension Causes Loss Of R/Neutral Short-Acting Effect. Endotext.org.
- ↑ 5.0 5.1 Huffman DM, Garber AJ. (1991). Availability of Soluble (R/Neutral) Insulin in Mixed Preparations With Crystalline (Lente) & Ultralente GE Insulin. Clinical Therapeutics. Cite error: Invalid
<ref>tag; name "Huffman" defined multiple times with different content - ↑ Lente-Type Insulins & NPH/Isophane Insulins-A Bad Combination. National Federation for the Blind.
- ↑ Havlik I, Galasko G, Alberts E, Furman KI, Seftel HC. (1988). Solubility Changes on Mixing Short- and Long-acting Insulin Preparations. South African Medical Journal.
- ↑ Deckert T. (1980). Intermediate-Acting Insulin Preparations: NPH (Isophane) & Lente. Diabetes Care.
Note--in 1980, there were no r-DNA/GE/GM insulins - ↑ Resource Guide. American Diabetes Association (2005).
- ↑ Hanas, Ragnar (1999). Insulin-Dependent Diabetes--Page 10. Children With Diabetes.
- ↑ Insulin Producers vs Doctors Re:Combining R/Neutral & Lente-type Insulins. Endotext.org.
- ↑ Bilo HJ, Heine RJ, Sikkenk AC, van der Meer J, van der Veen EA. (1987). Absorption Kinetics & Action Profiles-Single Subcutaneous Administration of Human Soluble (R/Neutral) & Lente Insulin. Diabetes Care.
- ↑ Heine RJ, Sikkenk AC, Eizenga WH, van der Veen EA. (1983). Delayed Onset of Action of Soluble (R/Neutral) Insulin After Premixing With Lente Insulin. Diabetes Research & Clinical Practice.
- ↑ 14.0 14.1 14.2 Insulin Therapy-Mixing Precautions. RxEd.org.
- ↑ Phenol Preservatives & Lente-type Insulins--A Bad Combination. National Federation for the Blind.
- ↑ Discussion of differences between r-DNA Ultralente and beef Ultralente insulins. Free Patents Online.
- ↑ Insulin Preparations. Committee on Safety in Medicines-UK (1976).
More information[]
- Lente Insulins-Injectable Suspensions West Virginia University College of Pharmacy-2009
- Randomized Controlled Clinical Trial of Glargine Versus Ultralente Insulin in the Treatment of Type 1 Diabetes Diabetes Care 2005

